Amlodipine, Valsartan, and Hydrochlorothiazide Tablet USP Assay - AppNote
January 21, 2025
/
/
/
/
/
/
/
Alternate L1 Column used for a USP Assay
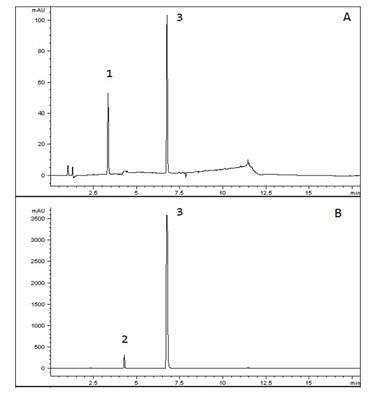
This AppNote demonstrates the USP Method for Amlodipine, Valsartan, and Hydrochlorothiazide Tablets working with the Cogent RP C18. As shown in the Chromatograms, the Peak efficiency is superb and USP Peak tailing guidelines are easily met with the Column. This demonstrates a great alternative Column for easy plug and play with this USP Method.

Attachment
N0.-381 Amlodipine, Valsartan, and Hydrochlorothiazide pdf 0.1 Mb Download File
This AppNote demonstrates the USP Method for Amlodipine, Valsartan, and Hydrochlorothiazide Tablets working with the Cogent RP C18. As shown in the Chromatograms, the Peak efficiency is superb and USP Peak tailing guidelines are easily met with the Column. This demonstrates a great alternative Column for easy plug and play with this USP Method.


Method Conditions:
Column: Cogent RP C18™ 3um, 100A
Catalog No.: 68318-15P
Dimensions: 4.6 mm x 150 mm
Sample Preparation:
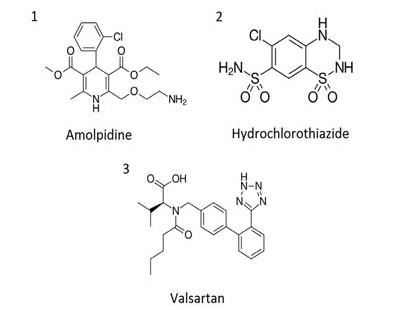
--A: Valsartan/Hydrochlorothiazide standards (0.16/0.025mg per mL)
--B: Valsartan/Amlopidine Sample (3.2/0.1mg per mL)

Attachment
N0.-381 Amlodipine, Valsartan, and Hydrochlorothiazide pdf 0.1 Mb Download File
