A Cannabis Metabolite in Urine Matrix Analyzed With LCMS - AppNote INTERNAL
March 13, 2019
/
/
/
/
/
THIS IS A DUPLICATE
Retention, Detection and Quantitation of a Cannabis Metabolite from Urine Samples
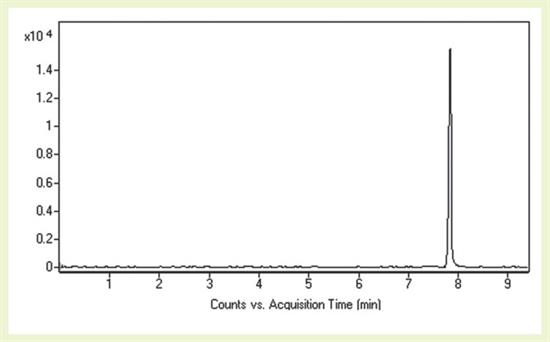
Using the SPE technique in "Sample Preparation" section of this AppNote, successful MS detection of a Cannabis Metabolite using the Cogent Phenyl Hydride™ HPLC Column is described.
It is worth noting that the retention of this compound was achieved using Methanol rather than Acetonitrile as the organic component in the Mobile Phase.
Method Conditions:
Column: Cogent Phenyl Hydride™ 4um, 100A
Catalog No.: 69020-05P-2
Dimensions: 2.1 50mm
Mobile Phase:
- A. DI Water / 0.1% Formic Acid (v/v)
- B. Methanol / 0.1% Formic Acid (v/v)
Gradient:
Post Time: 3 minutes
Injection Volume: 1ul
Flow Rate: 0.4ml / minute
Detection: ESI – POS – Agilent™ 6210 MSD TOF Mass Spectrometer
tο: 0.9 minutes
Sample Preparation: Urine sample was loaded into an SPE cartridge II (Clean Screen Xcel, UCT Bristol, PA, USA) and eluted with 1mL of Acetonitrile, 2-Propanol, Formic Acid (50/50/1). After elution, the sample was dried under Nitrogen gas and dissolved in 100 uL of 50% Methanol / 50% DI Water / 0.1% Formic Acid. The solution was filtered through a 0.45µm AQ™ brand Nylon Syringe Filter (MicroSolv Technology Corp.)
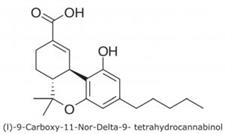
Note: (l)-9-Carboxy-11-Nor-Delta-9- THC is the main metabolite of Tetrahydrocannabinol (THC), formed in the body after consumption of Cannabis. The compound stays in the body for a significant time, making it useful as a test compound for Cannabis use. In the U.S., Cannabis is a controlled substance at the Federal level, although many states have recently enacted laws legalizing it.

Attachment
l-9-Carboxy-11-Nor-Delta-9- tetrahydrocannabinol.pdf 0.2 Mb Download File
Retention, Detection and Quantitation of a Cannabis Metabolite from Urine Samples
Using the SPE technique in "Sample Preparation" section of this AppNote, successful MS detection of a Cannabis Metabolite using the Cogent Phenyl Hydride™ HPLC Column is described.
It is worth noting that the retention of this compound was achieved using Methanol rather than Acetonitrile as the organic component in the Mobile Phase.


Peak:
(l)-9-Carboxy-11-Nor-Delta-9- THC, m/z 345.2060 [M+H]+
Method Conditions:
Column: Cogent Phenyl Hydride™ 4um, 100A
Catalog No.: 69020-05P-2
Dimensions: 2.1 50mm
Mobile Phase:
- A. DI Water / 0.1% Formic Acid (v/v)
- B. Methanol / 0.1% Formic Acid (v/v)
Gradient:
| Time (Minutes) | %B |
| 0 | 10 |
| 3 | 10 |
| 7 | 80 |
| 8 | 10 |
Post Time: 3 minutes
Injection Volume: 1ul
Flow Rate: 0.4ml / minute
Detection: ESI – POS – Agilent™ 6210 MSD TOF Mass Spectrometer
tο: 0.9 minutes
Sample Preparation: Urine sample was loaded into an SPE cartridge II (Clean Screen Xcel, UCT Bristol, PA, USA) and eluted with 1mL of Acetonitrile, 2-Propanol, Formic Acid (50/50/1). After elution, the sample was dried under Nitrogen gas and dissolved in 100 uL of 50% Methanol / 50% DI Water / 0.1% Formic Acid. The solution was filtered through a 0.45µm AQ™ brand Nylon Syringe Filter (MicroSolv Technology Corp.)
Note: (l)-9-Carboxy-11-Nor-Delta-9- THC is the main metabolite of Tetrahydrocannabinol (THC), formed in the body after consumption of Cannabis. The compound stays in the body for a significant time, making it useful as a test compound for Cannabis use. In the U.S., Cannabis is a controlled substance at the Federal level, although many states have recently enacted laws legalizing it.

Attachment
l-9-Carboxy-11-Nor-Delta-9- tetrahydrocannabinol.pdf 0.2 Mb Download File
