Bi-Est® Method Transfer - AppNote
July 23, 2015
/
/
/
/
/
4μm to Near-UHPLC – Separation of Hormones: Estriol, Estradiol, and Progesterone
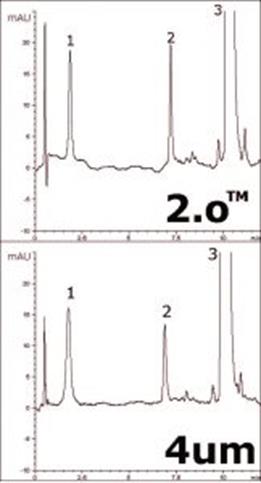
Separation of three components of a hormone replacement formulation is demonstrated in this Application Note using the Cogent Bidentate C18 2.o™ Column. The two figures demonstrate how comparable retention can be obtained for both the near-UHPLC column as well as the standard 4μm Column, allowing for easy method transfer.
As an example of the advantages for the 2.o™ Column, the calculated efficiency for peak 2 was 201,360 plates/m for the 4μm Column and 383,800 for the 2.o™.
Column: Cogent Bidentate C18 2.o™, 2.2μm, 120Å
Catalog No.: 40218-05P-2
Dimensions: 2.1 x 50 mm
Solvents:
--A: 90% DI Water / 10% Acetonitrile / 0.1% Formic Acid (v/v)
--B: Acetonitrile / 0.1% Formic Acid (v/v)
Post time: 5 minutes
Injection vol.: 2μL
Flow rate: 0.3mL/minute
Detection: UV @ 210 nm
Sample: The contents of a capsule containing 0.124 mg Estradiol, 1.001 mg Estriol, and 50 mg Progesterone were added to a 25 mL volumetric flask. The flask was diluted to mark with 5% solvent A / 95% solvent B and sonicated 10 min. Then a portion was filtered with a 0.45µm Nylon Syringe Filter (MicroSolv Tech. Corp.). Peak identities were confirmed by individual standards.

Attachment
No 345 Bi-Est Method Transfer pdf 0.3 Mb Download File
Separation of three components of a hormone replacement formulation is demonstrated in this Application Note using the Cogent Bidentate C18 2.o™ Column. The two figures demonstrate how comparable retention can be obtained for both the near-UHPLC column as well as the standard 4μm Column, allowing for easy method transfer.
As an example of the advantages for the 2.o™ Column, the calculated efficiency for peak 2 was 201,360 plates/m for the 4μm Column and 383,800 for the 2.o™.


Peaks:
1. Estriol
2. Estradiol
3. Progesterone
Column: Cogent Bidentate C18 2.o™, 2.2μm, 120Å
Catalog No.: 40218-05P-2
Dimensions: 2.1 x 50 mm
Solvents:
--A: 90% DI Water / 10% Acetonitrile / 0.1% Formic Acid (v/v)
--B: Acetonitrile / 0.1% Formic Acid (v/v)
| Time (minutes) | %B |
| 0 | 20 |
| 2 | 20 |
| 11 | 80 |
| 12 | 20 |
Injection vol.: 2μL
Flow rate: 0.3mL/minute
Detection: UV @ 210 nm
Sample: The contents of a capsule containing 0.124 mg Estradiol, 1.001 mg Estriol, and 50 mg Progesterone were added to a 25 mL volumetric flask. The flask was diluted to mark with 5% solvent A / 95% solvent B and sonicated 10 min. Then a portion was filtered with a 0.45µm Nylon Syringe Filter (MicroSolv Tech. Corp.). Peak identities were confirmed by individual standards.

Attachment
No 345 Bi-Est Method Transfer pdf 0.3 Mb Download File
