Pepto-Bismol® Tablet Method Transfer with HPLC - AppNote
June 10, 2015
/
/
/
/
/
INTENAL ONLY- Bismuth Subsalicylate and Salicylic Acid cannot be separated
A Simple Assay Method for the widely used Pepto-Bismol® Formulation
Excellent Separation of Bismuth Subsalicylate and Salicylic Acid is achieved showing that this Method can be readily transferred to a near-UHPLC 2.o phase (Figure A) from a standard 4μm Column (Figure B).
The same Column Dimensions are used in both cases, with the only difference being the particle size. The analyte Retention Times of both Peaks are highly consistent between the two Columns, allowing for easy Method Transfer. In terms of theoretical plates, the smaller particle size resulted in a measured 50% increase in Efficiency.
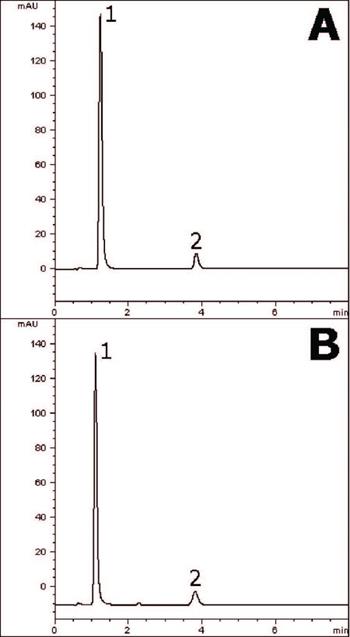
Column: Cogent Bidentate C18 2.o™, 2.2μm, 120Å
Catalog No.: 40218-05P-2
Dimensions: 2.1 x 50mm
Mobile Phase: 65:35 DI Water / Acetonitrile / 0.1% Formic Acid (v/v)
Injection vol.: 1.0µL
Flow rate: 0.3mL / minute
Detection: UV @ 302nm
Sample Preparation: Pepto-Bismol® tablet (containing 262mg Bismuth Subsalicylate and 102mg Salicylate) was ground and added to a 100mL volumetric flask containing a portion of 1:1 Acetonitrile / DI Water. Then it was sonicated 10 minutes and diluted to mark. After mixing the flask, a portion was filtered (0.45μm, Nylon, MicroSolv Tech. Corp.) and used for HPLC injections. The Salicylic Acid Peak was identified with a reference standard.
Note: Pepto-Bismol® is primarily used as an antacid and to alleviate upset stomach. Originally called “Mixture Cholera Infantum” by the doctor who first invented it, the formulation was later modified, re-branded, and manufactured as Pepto-Bismol® by the Norwich Pharmacal Company.

Attachment
No 340 Pepto-Bismol® Tablet Method Transfer with HPLC pdf 0.2 Mb Download File
A Simple Assay Method for the widely used Pepto-Bismol® Formulation
Excellent Separation of Bismuth Subsalicylate and Salicylic Acid is achieved showing that this Method can be readily transferred to a near-UHPLC 2.o phase (Figure A) from a standard 4μm Column (Figure B).
The same Column Dimensions are used in both cases, with the only difference being the particle size. The analyte Retention Times of both Peaks are highly consistent between the two Columns, allowing for easy Method Transfer. In terms of theoretical plates, the smaller particle size resulted in a measured 50% increase in Efficiency.

Peaks:
1. Salicylic Acid
2. Bismuth Subsalicylate
Column: Cogent Bidentate C18 2.o™, 2.2μm, 120Å
Catalog No.: 40218-05P-2
Dimensions: 2.1 x 50mm
Mobile Phase: 65:35 DI Water / Acetonitrile / 0.1% Formic Acid (v/v)
Injection vol.: 1.0µL
Flow rate: 0.3mL / minute
Detection: UV @ 302nm
Sample Preparation: Pepto-Bismol® tablet (containing 262mg Bismuth Subsalicylate and 102mg Salicylate) was ground and added to a 100mL volumetric flask containing a portion of 1:1 Acetonitrile / DI Water. Then it was sonicated 10 minutes and diluted to mark. After mixing the flask, a portion was filtered (0.45μm, Nylon, MicroSolv Tech. Corp.) and used for HPLC injections. The Salicylic Acid Peak was identified with a reference standard.
Note: Pepto-Bismol® is primarily used as an antacid and to alleviate upset stomach. Originally called “Mixture Cholera Infantum” by the doctor who first invented it, the formulation was later modified, re-branded, and manufactured as Pepto-Bismol® by the Norwich Pharmacal Company.

Attachment
No 340 Pepto-Bismol® Tablet Method Transfer with HPLC pdf 0.2 Mb Download File
