Tizanidine HCl Tablet Analyzed by HPLC - AppNote
January 24, 2013
/
/
/
/
/
Separation Method for Tizanidine Compatible with LC-MS
Tizanidine has numerous amine functional groups and can be a challenge for analysis by HPLC. The USP method uses Phosphate in the Mobile Phase which is not compatible with LC-MS. This Method however uses Formic Acid as the Mobile Phase additive and produces a sharp, symmetrical peak.
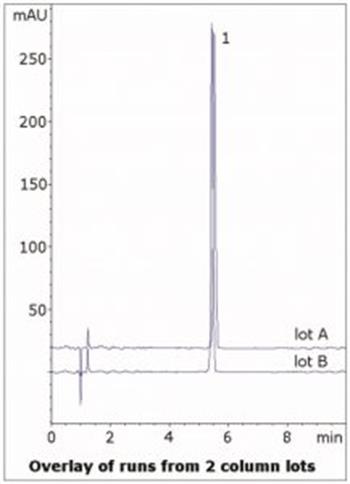
The USP system suitability for the tailing factor is not more than 1.6, and the Peak obtained has a value of 1.1. Data from two Column lots is shown in the figure, demonstrating Robustness of this Method.
Column: Cogent Diamond Hydride™, 4µm, 100Å
Catalog No.: 70000-7.5P
Dimensions: 4.6 x 75mm
Mobile Phase:
--A: DI Water / 0.1% Formic Acid (v/v)
--B: Acetonitrile / 0.1% Formic Acid (v/v)
Gradient:
Post Time: 3 minutes
Injection vol.: 1µL
Flow rate: 1.0mL / minute
Detection: UV @ 230nm
Sample Preparation: 4mg strength Tizanidine HCL tablet was ground and weighed in a 10mL volumetric flask. A portion of 50:50 Solvent A / Solvent B diluent was added and the flask was sonicated 10 minutes. It was then diluted to mark and filtered with a 0.45µm Nylon Syringe Filter (MicroSolv Tech Corp.).
t0: 0.9 minutes
Note: Tizanidine is a centrally acting a2-adrenergic agonist used to treat spasms, cramping, tightness of muscles, and related conditions. It is available under the trade name Zanaflex® as well as generic versions.

Attachment
No 232 Tizanidine HCL Tablet Analyzed by HPLC pdf 0.6 Mb Download File
Tizanidine has numerous amine functional groups and can be a challenge for analysis by HPLC. The USP method uses Phosphate in the Mobile Phase which is not compatible with LC-MS. This Method however uses Formic Acid as the Mobile Phase additive and produces a sharp, symmetrical peak.
The USP system suitability for the tailing factor is not more than 1.6, and the Peak obtained has a value of 1.1. Data from two Column lots is shown in the figure, demonstrating Robustness of this Method.


Peak:
Tizanidine HCI
Column: Cogent Diamond Hydride™, 4µm, 100Å
Catalog No.: 70000-7.5P
Dimensions: 4.6 x 75mm
Mobile Phase:
--A: DI Water / 0.1% Formic Acid (v/v)
--B: Acetonitrile / 0.1% Formic Acid (v/v)
Gradient:
| Time (minutes) | %B |
| 0 | 95 |
| 1 | 95 |
| 6 | 40 |
| 7 | 95 |
Injection vol.: 1µL
Flow rate: 1.0mL / minute
Detection: UV @ 230nm
Sample Preparation: 4mg strength Tizanidine HCL tablet was ground and weighed in a 10mL volumetric flask. A portion of 50:50 Solvent A / Solvent B diluent was added and the flask was sonicated 10 minutes. It was then diluted to mark and filtered with a 0.45µm Nylon Syringe Filter (MicroSolv Tech Corp.).
t0: 0.9 minutes
Note: Tizanidine is a centrally acting a2-adrenergic agonist used to treat spasms, cramping, tightness of muscles, and related conditions. It is available under the trade name Zanaflex® as well as generic versions.

Attachment
No 232 Tizanidine HCL Tablet Analyzed by HPLC pdf 0.6 Mb Download File
