EDTA Analysis with HPLC - AppNote
October 3, 2020
/
/
/
/
/
/
EDTA does not have a significant Chromophore, so to achieve UV Detection, in the Method shown below we used a pre-Column reaction of a Solution of Ferric Chloride with the Sample. The resulting EDTA/Fe3+ has significant UV Absorbance making this a very Sensitive Method.
Ethylenediaminetetraacetic acid is extremely difficult to analyze by itself however in its complexed form, it chromatographs well from matrices such as river sediment and other solutions.
Method Conditions:
Column: Cogent HPS C8™, 5μm, 120Å
Catalog No.: 75008-15P
Dimensions: 4.6 x 150mm
Mobile Phase: 98:2 DI H2O/ Acetonitrile with 0.1% Acetic Acid (pH 3.5/2gL Tetrabutylammonium Sulfate)
Temperature: 40°C
LOQ: 0.2μg / mL
Injection vol.: 20μL
Flow rate: 2mL / minute
Note: EDTA is a synthetic metal complexing reagent that is used in a wide variety of industrial applications. Used a preservative, it has very low biodegradability thus remains in the environment for long periods of time. Found in sewer water, freshwater and ground water, it re-solubilizes precipitated toxic metals back into solution where they can be ingested by plants and animals.

Attachment
A74. EDTA Analysis with HPLC pdf 8.7 Kb Download File
Ethylenediaminetetraacetic acid is extremely difficult to analyze by itself however in its complexed form, it chromatographs well from matrices such as river sediment and other solutions.

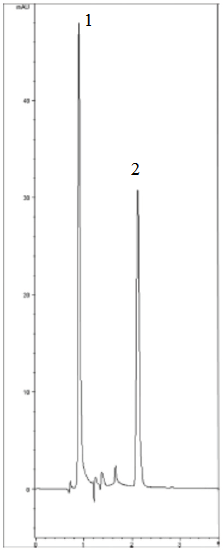
Peaks:
1. Water (solvent front)
2. EDTA Fe3+

Method Conditions:
Column: Cogent HPS C8™, 5μm, 120Å
Catalog No.: 75008-15P
Dimensions: 4.6 x 150mm
Mobile Phase: 98:2 DI H2O/ Acetonitrile with 0.1% Acetic Acid (pH 3.5/2gL Tetrabutylammonium Sulfate)
Temperature: 40°C
LOQ: 0.2μg / mL
Injection vol.: 20μL
Flow rate: 2mL / minute
Note: EDTA is a synthetic metal complexing reagent that is used in a wide variety of industrial applications. Used a preservative, it has very low biodegradability thus remains in the environment for long periods of time. Found in sewer water, freshwater and ground water, it re-solubilizes precipitated toxic metals back into solution where they can be ingested by plants and animals.

Attachment
A74. EDTA Analysis with HPLC pdf 8.7 Kb Download File
