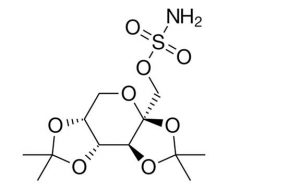
Retention of the Sulfonate Drug Topiramate
The structure of Topiramate leads us to think due to the Amino group and negative Log P value of -0.8, it will not be retained in Reversed Phase HPLC. However, in this Method, the compound is well retained.
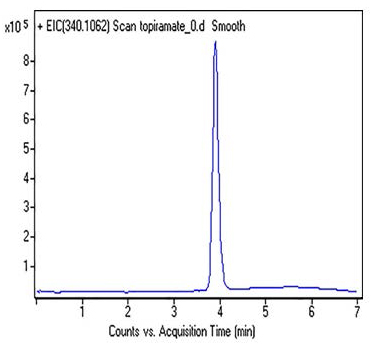
Peak:
Topiramate 340.1062 m/z (M + H)+
Method Conditions:
Column: Cogent Bidentate C18™, 4µm, 100Å
Catalog No.: 40018 -05P-2
Dimensions: 2.1 x 50mm
Mobile Phase:
—A: DI Water / 0.1% Formic Acid
—B: Acetonitrile / 0.1% Formic Acid
Gradient:
| Time (minutes) | %B |
| 0 | 10 |
| 1 | 10 |
| 4 | 90 |
| 5 | 90 |
| 6 | 10 |
| 7 | 10 |
Injection vol.: 1µL
Flow rate: 0.3mL / minute
Detection: ESI – pos – Agilent 6210 MSD TOF Mass Spectrometer
Sample Preparation: Topiramate 0.01mg / mL in 50% A / 50% B solvent mixture.
Notes: Migraines are a common, disabling neurological disorder and are often accompanied by one or more of the following disabling symptoms: visual disturbances, nausea, vomiting, dizziness, extreme sensitivity to sound, light, touch and smell, and tingling or numbness in the extremities or face. Migraines involve a complex interchange between various brain regions, including the hypothalamus and brainstem nuclei that modulate pain signaling. The headache phase involves activation of the Trigeminovascular system. Topiramate modulates the Trigeminovascular signaling that is effective in migraine prevention. It is also used as an Antiepileptic drug (AED) and is structurally distinct from other AEDs as it is derived from D-fructose, a naturally occurring sugar moiety, and has sulfamate functionality
Attachment
A377 Retention of a Sulfonate Drug by LCMS pdf 0.1 Mb Download File


