Separation of API from Matrix Peaks from an Over The Counter Tablet
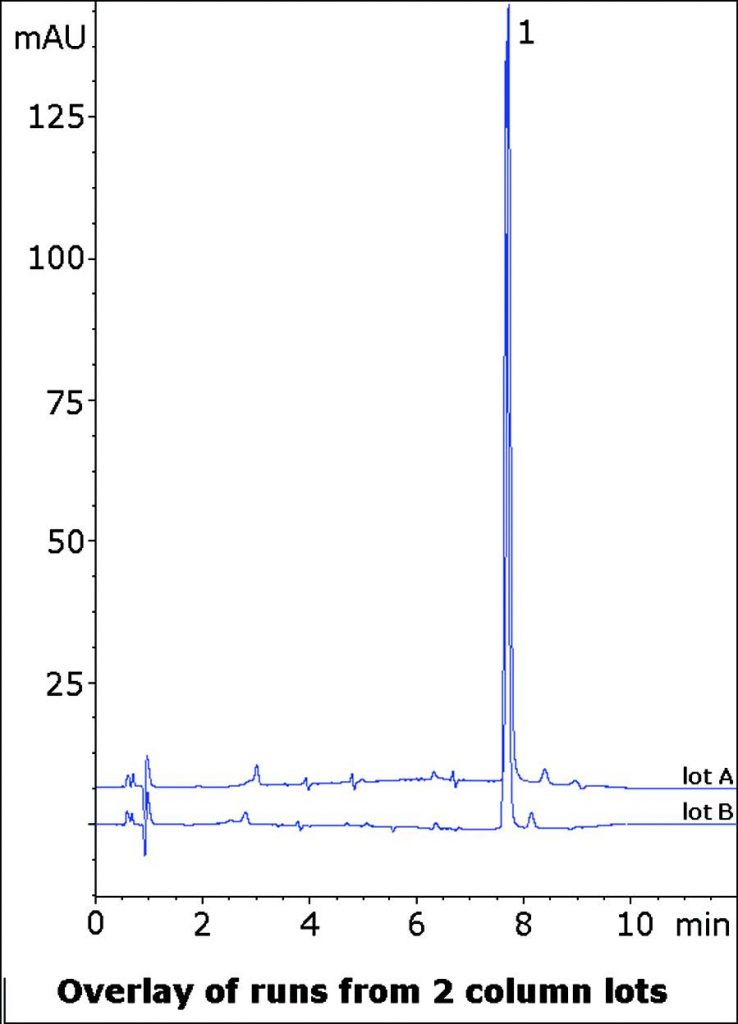
This Method for Analysis of a Bisacodyl Tablet formulation Separates the API from several small matrix Peaks. These Peaks must be Resolved from the main Peak in order to ensure accurate Quantitation. The data below illustrates the separation potential of the this Method for Assay or Impurity Methods. Ammonium Acetate was used in the Mobile Phase and extraction Solvent to avoid any acid-catalyzed hydrolysis of the Bisacodyl ester groups.
Runs from two Column lots are shown demonstrating consistency and Robustness of this Method.
Peak:
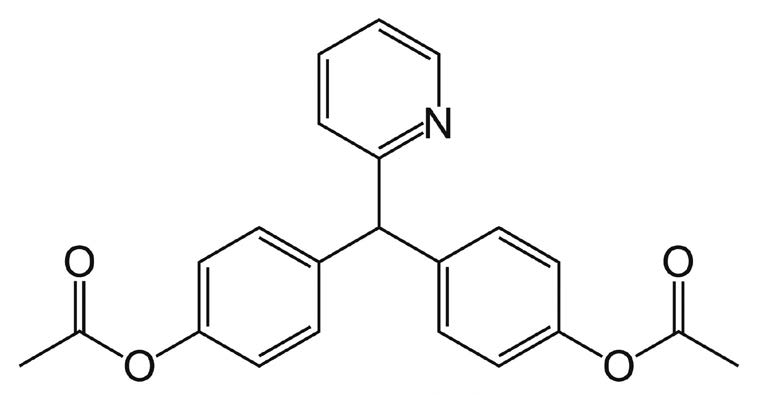
Bisacodyl
Method Conditions
Column: Cogent Bidentate C18™, 4μm, 100Å
Catalog No.: 40018-75P
Dimensions: 4.6 x 75mm
Mobile Phase:
—A: DI Water with 10mM Ammonium Acetate
—B: 95:5 Acetonitrile / Solvent A (v/v)
Gradient:
| Time (minutes) | %B |
| 0 | 20 |
| 1 | 20 |
| 6 | 60 |
| 8 | 60 |
| 9 | 20 |
Post Time: 3 minutes
Injection vol.: 2μL
Flow rate: 1.0mL / minute
Detection: UV @ 254nm
Sample Preparation: 5mg strength Bisacodyl Tablet was ground and added to a 10mL volumetric flask containing a portion of Solvent B. It was then sonicated 10 minutes and diluted to mark. After mixing, a portion was filtered with a 0.45μm Nylon Syringe Filter (MicroSolv Tech Corp.).
t0: 0.9 minutes
Note: Bisacodyl is a stimulant laxative used in many common over-the-counter formulations. Brand names include Dulcogen®, Alophen®, and Dulcolax®.
Attachment
No 227 Bisacodyl Tablet Analyzed with HPLC pdf 0.3 Mb Download File