Analyte Ionization using L-Tyrosine
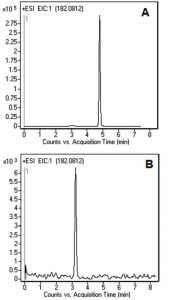
Figures A and B show the Peak for L-Tyrosine obtained with two LCMS Methods. The analysis shown in Figure B was performed with a stronger ANP Solvent (DI Water) as component A of the Mobile Phase. The obtained Retention time was lower than in Figure A, as was the compound ionization.
An eluent containing 2-Propanol is a better choice for the analysis of this compound, since the ionization of L-Tyrosine is greater. In addition, the Solvents used in Figure A are recommended when biological samples are analyzed. As such, the Method can be directly transferred to the analysis of L-Tyrosine in plasma samples for monitoring of patients with Phenyloketonuria.
 Peak:
Peak:
L-Tyrosine 182.0812 m/z
Method Conditions
Column: Cogent Diamond Hydride™, 4µm, 100Å
Catalog No.: 70000-15P-2
Dimensions: 2.1 x 150mm
Mobile Phase:
Figure A
—A: 50% DI Water / 50% 2-Propanol / 0.1% Formic Acid v/v
—B: Acetonitrile / 0.1% Formic Acid v/v
Figure B
—A: DI Water / 0.1% Formic Acid v/v
—B: Acetonitrile / 0.1% Formic Acid v/v
Gradient:
| Time (minutes) | %B |
| 0 | 90 |
| 4 | 30 |
| 6 | 30 |
| 7 | 90 |
Post Time: 3 minutes
Injection vol.: 1µL
Flow rate: 0.4mL / minute
Detection: ESI – POS – Agilent 6210 MSD TOF Mass Spectrometer
Sample Preparation: 500mg L-Tyrosine capsule contents were dissolved in 500mL of 50:50 Water / Acetonitrile. The solution was shaken for 20 minutes and filtered through a disposable 0.45µm Nylon Syringe Filter (MicroSolv Tech Corp.). The sample for injection was diluted 1:100 with a 50:50 Solvent A / Solvent B mixture.
t0: 0.9 minutes
Note: ANP Methods have an inherent advantage in LCMS over Reversed Phase Methods in terms of Sensitivity. This is because Acetonitrile is more volatile than Water and hence more readily removed in LCMS, and ANP Methods typically use a higher percentage of Acetonitrile in the Mobile Phase compared to Reversed Phase Methods.


