Alternative to USP Method with Improved Peak Efficiency
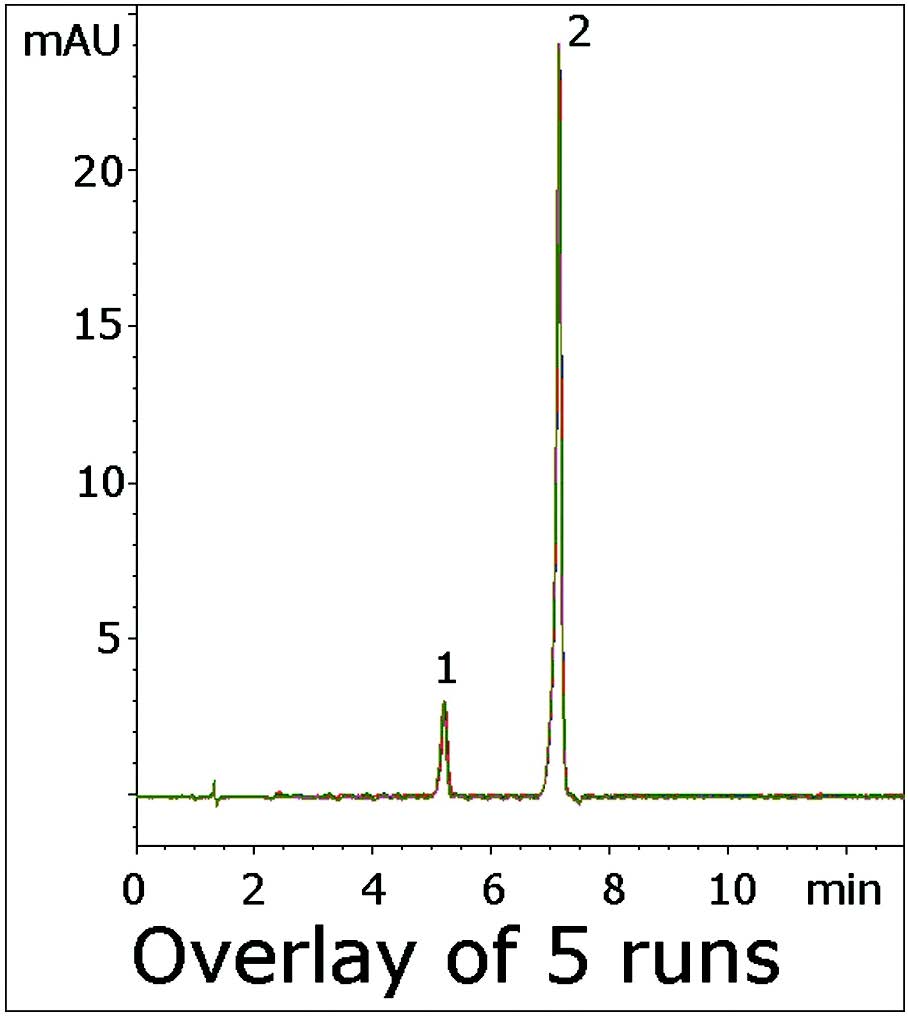
The USP impurities Method for Verapamil uses an Acetate Buffer with the ion pair agent 2-Aminoheptane to reduce Peak Tailing of the API and its Impurity. This Application Note uses an LCMS compatible Mobile Phase and produces Symmetrical Peaks for both Compounds. Excellent Resolution was also obtained between the two Peaks, far exceeding the USP System Suitability of Rs≥1.5.
This Method is a significant improvement over the USP monograph and illustrates the considerable potential for analyses of Amine containing solutes.
Peaks:
1. Verapamil Related Compound B
2. Verapamil
Method Conditions
Column: Cogent Diamond Hydride™, 4μm, 100Å
Catalog No.: 70000-7.5P
Dimensions: 4.6 x 75mm
Mobile Phase:
—A: DI Water / 10mM Ammonium Formate
—B: 95:5 Acetonitrile / 10mM Ammonium Formate (v/v)
Gradient:
| Time (minutes) | %B |
| 0 | 100 |
| 9 | 85 |
| 10 | 100 |
Post Time: 4 minutes
Injection vol.: 1μL
Flow rate: 1.0mL / minute
Detection: UV @ 278nm
Sample Preparation: Sample mixture contained 0.1mg / mL Verapamil and 0.02mg / mL Verapamil Related Compound B in a diluent of 50:50 Solvent A / Solvent B. Peak identities were confirmed with individual Standards.
t0: 0.9 minutes
Note: Verapamil is an L-type calcium channel blocker used in the treatment of Hypertension, Angina Pectoris, and Cardiac Arrhythmia.
Attachment
No 180 Verapamil and Impurity Analyzed with HPLC pdf 0.5 Mb Download File