Simple Isocratic Assay Method without Amine Additives
Click HERE for Column Ordering Information.
Fexofenadine, antihistamine, has a number of aromatic moieties and the tertiary amine in its chemical structure that can cause Peak tailing issues when using most L11 Columns for Analysis. The USP Assay Monograph for Fexofenadine Tablets uses Triethylamine to reduce tailing.
With this Method, Trifluoroacetic Acid is used instead and good Peak shapes are observed, as the five Chromatograms that are overlaid in the Figure show.
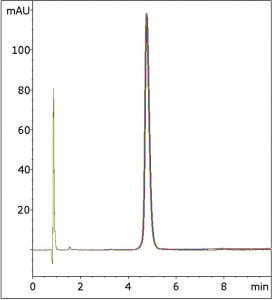
Peak:
Fexofenadine
Method Conditions
Column: Cogent Phenyl Hydride™, 4μm, 100Å
Catalog No.: 69020-7.5P
Dimensions: 4.6 x 75mm
Mobile Phase: 62% DI Water / 38% Acetonitrile / 0.1% Trifluoroacetic Acid (TFA)
Temperature: 35˚C
Flow rate: 1.0mL / minute
Detection: UV @ 220nm
Injection vol.: 1μL
Sample Preparation:
—Stock Solution: 180mg strength Allegra® (Fexofenadine) tablet was ground and added to a 100mL volumetric flask. It was diluted with the Mobile Phase and vortexed 5 minutes. A portion was filtered with a 0.45µm Nylon Syringe Filter (MicroSolv Tech Corp.).
—Working Solution: A 100µL aliquot of the Stock Solution was diluted with 900µL of the Mobile Phase.
t0: 0.87 minutes
Note: Fexofenadine is marketed under the trade name Allegra, but generic versions are also available. In January 2011, the Food and Drug Administration approved over-the-counter sales of the drug without a prescription. It is a widely selling antihistamine used for treatment of hay fever and other allergies. Because it does not cross the blood-brain barrier, it causes less drowsiness than first generation antihistamines.
Attachment
No 139 Fexofenadine Analyzed with HPLC pdf 0.3 Mb Download File


