Polar and Non-Polar Anticancer Prodrugs Analyzed in the Same Run
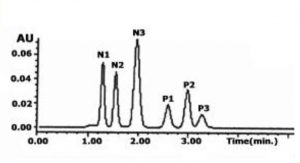
In this Method, we display Retention of both polar and nonpolar compounds in a simple Isocratic run. Adjustment of the aqueous to organic ratio can be used to make either the polar or more hydrophobic compounds elute first.
In the example presented, the more hydrophobic compounds are retained longer but retention and separation of the polar constituents is still achieved. If greater retention of the polar compounds is desired then a higher percentage of Acetonitrile is required.
Method Conditions
Column: Cogent UDC Cholesterol™, 4μm, 100Å
Catalog No.: 69069-7.5P
Dimensions: 4.6 x 75mm
Mobile Phase: 60% Acetonitrile / 40% DI Water / 0.5% Formic Acid
Injection vol.: 5μL
Flow rate: 1 mL / minute
Detection: UV @ 270nm
Sample Preparation: Proprietary Drugs. N1-N3 Nucleoside Analogues. P1-P3 have greater hydrophobicity with polar functional groups. 1µg / mL of each dissolved in the Mobile Phase
Notes: Nucleoside analogue anticancer “pro” drugs are difficult to retain on conventional HPLC Columns because of their highly polar natures. As such, Ion Pair Reagents are used in order to achieve sufficient Retention. These additives interfere with MS detection and are not preferred. With this Method, you can achieve good Retention paired with a MS-Compatible Mobile Phase.
Attachment
No 05 Nucleoside Analogs Analyzed with HPLC pdf 0.1 Mb Download File


